Biomaterials have revolutionized strategies to combat these life-threatening diseases; from tools to better understand disease development to tissue engineering strategies and drug delivery platforms. Biomaterial scaffolds are highly tailorable platforms that can be engineered to interact with existing tissue, mimic native tissue including cellular interactions with extracellular matrix molecules, and even alter cellular behavior. The main goal of our research is to develop biomaterial scaffolds to investigate and mimic cellular interactions with their environment and to harness this information to develop therapies for life-threatening diseases. Our research primarily focuses on type 1 diabetes (T1D), which is characterized by the progressive destruction of the insulin-producing β-cells in the pancreatic islets of Langerhans. This research combines novel biomimetic scaffold design with advanced live-cell imaging techniques to provide quantitative insight into pancreatic islet function and disease development. The discoveries that we make will ultimately help to improve therapies for patients with T1D.
Some of the projects we are currently working on include:
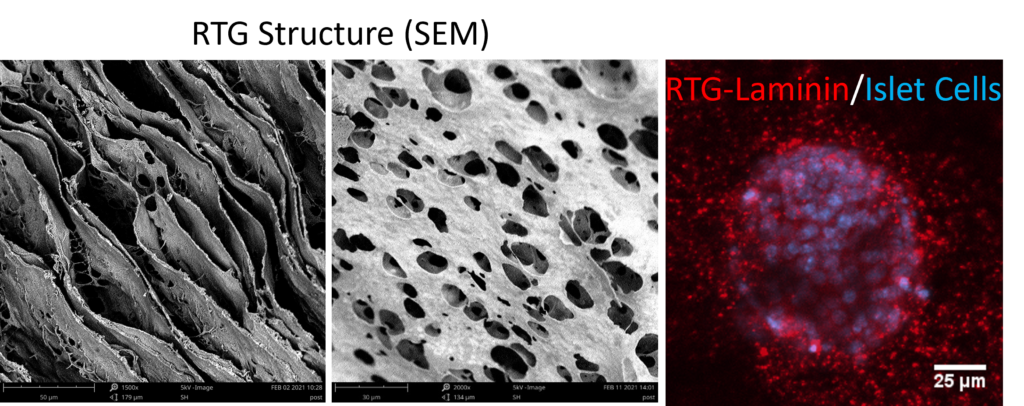
1. Developing a 3D biomimetic scaffold to determine the role of extracellular matrix interactions in cellular death and dysfunction during the onset and progression of type 1 diabetes. This project utilizes a reverse thermal gel, which forms a 3D scaffold at body temperature, with extracellular matrix molecules incorporated into the scaffold as a culture system to probe the function and survival of isolated pancreatic islets. This project will investigate the role of laminin-10 and collagen IV in regulating insulin secretion and islet survival in conditions similar to those found in T1D. The results from this project will determine if the pancreatic extracellular matrix plays a role in the onset and progression of T1D and will provide an in vitro culture system that can be used for screening of therapeutics to preserve the pancreatic islets in T1D.
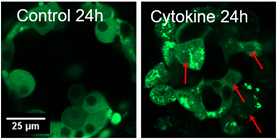
2. Investigating cellular mechanisms of β-cell death in the pancreatic islet in type 1 diabetes. This project combines quantitative live-cell fluorescence imaging with experimental biology to investigate novel mechanisms of β-cell death in the pancreatic islet in T1D. This project is mainly focused on a recent novel target that we have identified, protein kinase C δ (PKCδ) and determining the role PKCδ plays in mediating β-cell death. We are currently using fluorescent biosensors to determine how pro-inflammatory cytokines, which simulated the conditions found in T1D, changes the localization and activity of PKCδ in isolated mouse and human pancreatic islets. The results from this project will identify a novel regulator of β-cell death which can be targeted to prevent β-cell death in both patients with established disease and those at risk for developing T1D.
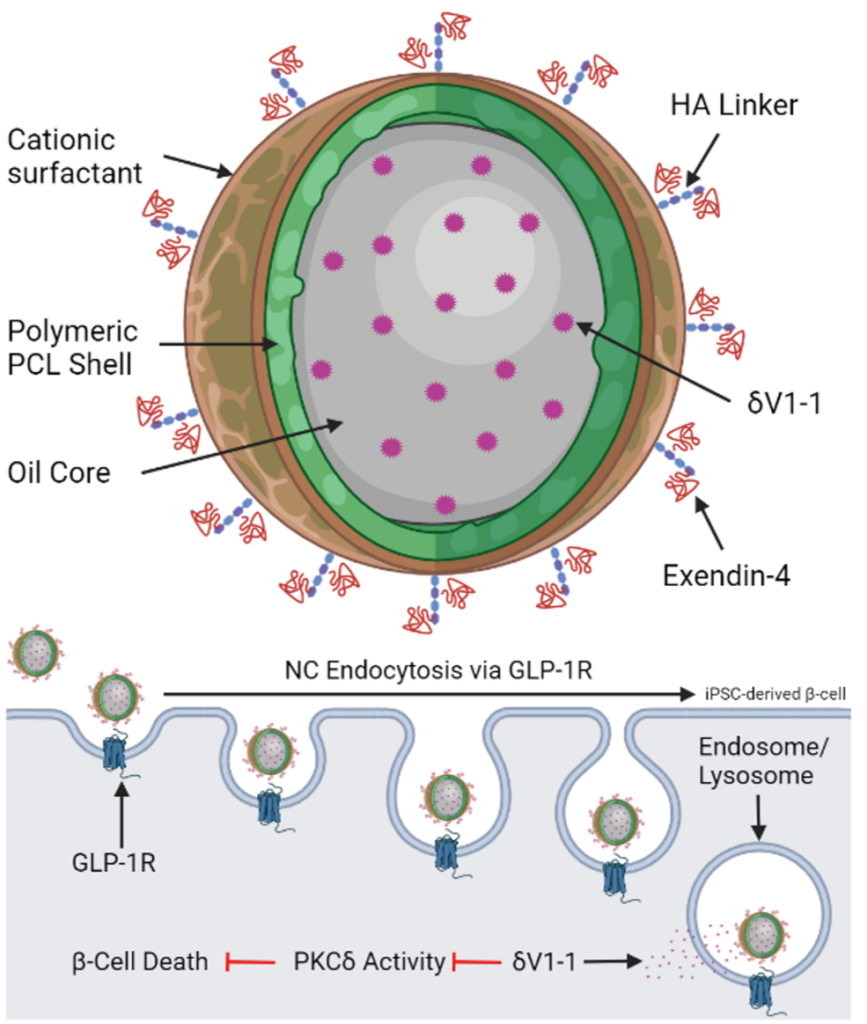
3. Developing drug delivery strategies to prevent pancreatic islet dysfunction and death during the pathogenesis of type 1 diabetes. This goal of this project is to develop drug-loaded nano-particles that can be targeted to the β-cell in the pancreatic islet to prevent dysfunction and death associated with T1D. Specifically, this project will use δV1-1, a highly selective inhibitor of PKCδ, loaded into β-cell targeted nano-particles to determine if we can prevent β-cell death in T1D. This project combines biomaterial design to optimize drug release, studies with human tissue from organ donors, and studies in transgenic mice that develop T1D. The results from this project will determine the efficacy of this therapy and feasibility of translating this therapy to human subjects.