Policy & Procedures
Mines employees and students engaged in approved Human Subjects Research are responsible for conducting the Research in accordance with the approved Research protocol, and in compliance with all applicable Mines policies and State and Federal regulations governing Human Subjects Research.
POLICY
Mines Human Subjects Research Protection Policy and Procedure resides in the Mines Policy Library.
REVIEW & APPROVAL
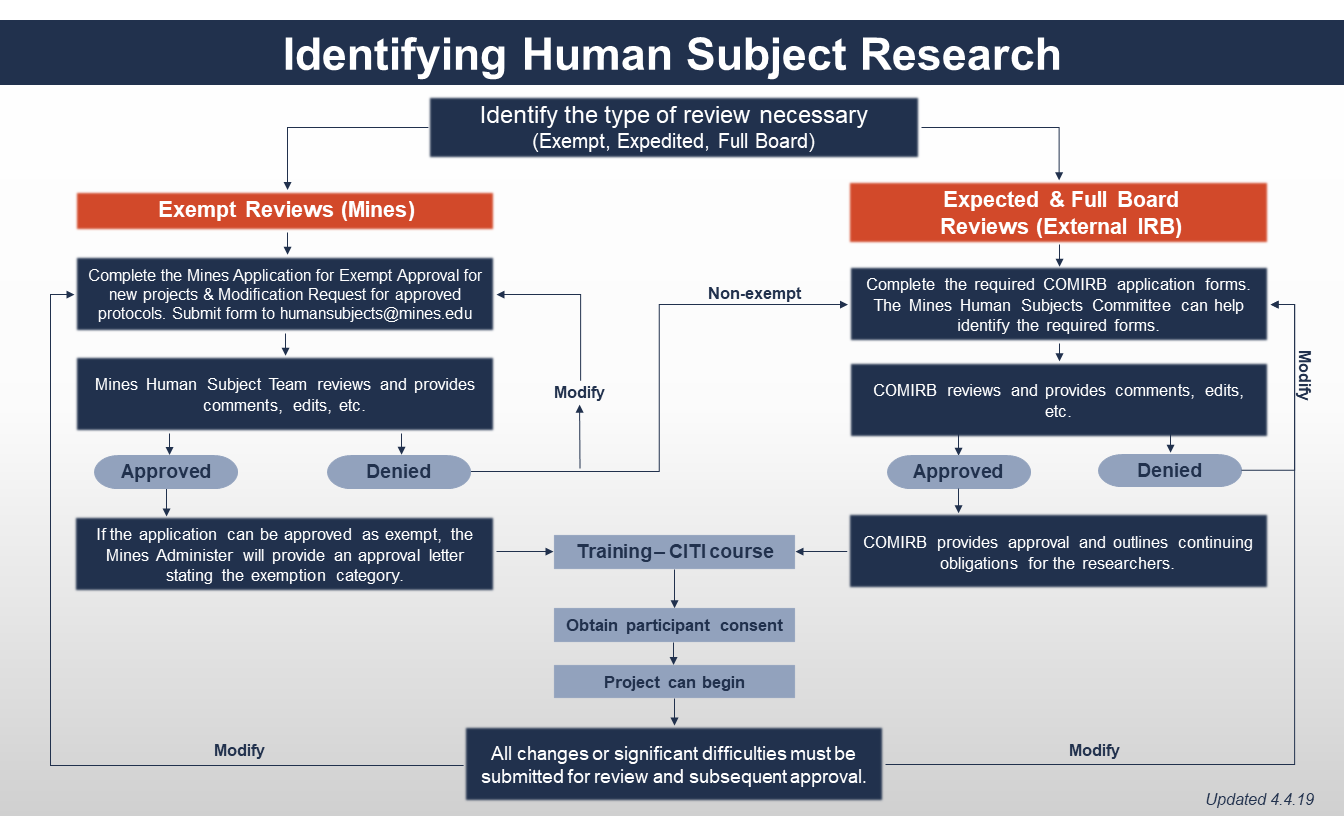
All exempt Research activities involving Human Subjects or data containing Private Information must be reviewed in advance by the Mines Human Subjects Committee and must be approved in advance by the Mines Human Subjects Administrator, or in their absence, the Vice President for Research and Technology Transfer (VPRTT). This is true regardless of whether an investigator believes his or her Human Subjects Research project qualifies for exemption under the Common Rule. Mines can only approve Exempt Human Subjects Research. Mines is not registered with the Office of Human Research Protection (OHRP) for a full internal IRB. Human Subjects Research protocols requiring an expedited approval or full board approval must be sent to an external IRB in good standing with OHRP.
Prior to submission for the Human Subjects Committee review, department head signature is required to acknowledge that the proposed study meets departmental research standards and provide assurance that the principal investigator will meet institutional requirements for review and approval of the research.
Required Training
All Mines employees and students must complete online training on Social & Behavioral Research before beginning any work on an approved Human Subjects Research project. Additional training requirements and review obligations may be necessary for projects requiring expedited or full IRB review.
Reportable Events
Any reportable events must be reported to the VPRTT within five (5) business days of discovery of the incident. The VPRTT or authorized delegate will review the reportable event and determine if it warrants further investigation. Reportable events may include, but are not limited to:
- An actual unforeseen, harmful or unfavorable occurrence to participants or others that relates to the Research protocol;
- A problem involving data collection, data storage, privacy or confidentiality. (Any violations of privacy or confidentiality also need to be reported to the Privacy Compliance Director)
- A protocol violation (meaning an accidental or unintentional change to the Mines approved protocol) that harms participants or others; or that indicates participants or others may be at increased risk of harm;
- Any study related event that requires prompt reporting to the Research sponsor; or
- Any other problem that creates a risk to the participant or others.