Poster Presentation by Kacey Kim
Senior, Chemical and Biological Engineering
Mentor: Stephanie Kwon, Chemical and Biological Engineering
Abstract:
This project aims to identify different types of active oxygen species formed on PdO surfaces via H2O2 activation and to understand their role in selective oxidation of alkanes and alkenes. Using cutting-edge density-functional theory (DFT) calculations combined with statistical mechanical treatments, we calculated free energies of all plausible H2O2-derived species on PdO surfaces and of intermediates and transition states involved in their reactions with light alkanes (CH4, C2H6, C3H8) and alkenes (C2H4, C3H6). We expect that results of this study will provide essential knowledge in selective oxidation catalysis that involve electrophilic oxygen species, which will ultimately allow us to design reactive and selective catalytic system for light alkane/alkene upgrading processes.
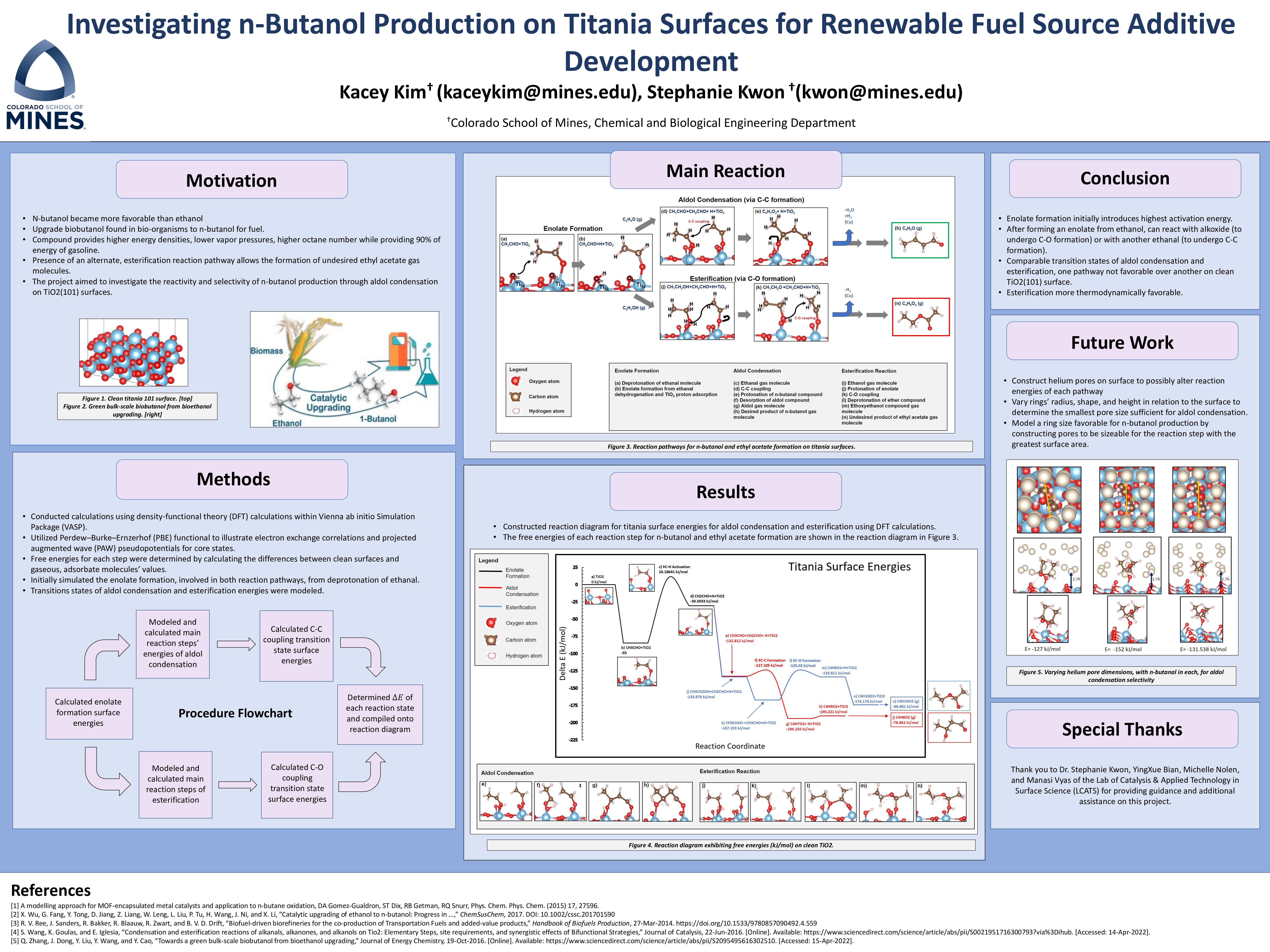
I also had the opportunity to investigate the reactivity and selectivity of n-butanol production through aldol condensation on TiO2 surfaces. This surface was determined to be used in this study as the material is a good metal oxide catalyst support due to the compound’s characteristics. Theoretical analysis of the reactivity of each pathway was conducted using cutting-edge density- functional theory (DFT) calculations, using PBE-D2/PAW, with statistical mechanical treatments. Through these computational simulations, I was able to calculate the free energies of all plausible enolate-derived species on TiO2 surfaces and of intermediates and transition states involved in their reactions with ethanal or ethanol. I expected that results of this study will provide further insight into optimization of respective adsorbate’s geometry for greater selectivity and reactivity, which will ultimately allow us to design reactive and selective catalytic system for ethanol upgrading processes for n- butanol production.